Atrial Fibrillation
Detecting biomarkers of early Atrial Transformation in AF
Study Scope and Protocol
This large scale 3,000 patient AF study spans up to 10 years, and is designed to detect biomarkers of early atrial transformation in atrial fibrillation. Participants are required to apply a wearable ECG monitor for one week durations every month or quarter, in ambulatory and home settings.
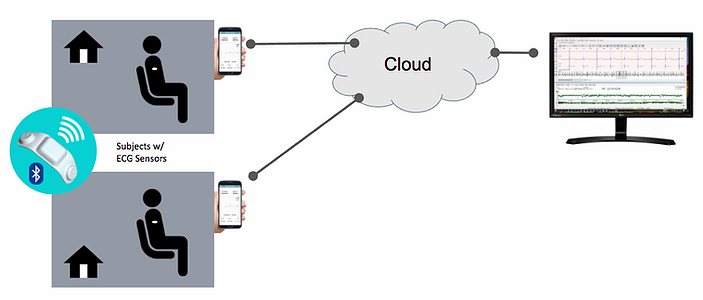
During the study, the Pulsebridge Biometrics Data Platform and continuous ECG monitor were used for remote patient monitoring to capture the ECG rhythm, integrated into a mobile patient app, and then transmitted it to the cloud. Data from the cloud was then sent to an arrhythmia detection algorithm for retrospective analysis and reporting.
Key Components
- Biometrics Data Platform
- Wearable ECG Monitor
- Custom Mobile Patient App
- Deeplink Integration
- Arrhythmia Detection Algorithm
- Retrospective Analysis Report
Data Captured
- Raw ECG rhythm
- Heart rate
- RR-interval
| Requirements/Challenges | Solution/Benefits |
| Must be patient self-serviceable | ECG patch designed for self-services |
| Network disruptions in ambulatory settings | Onboard memory with auto network data sync |
| Integrated clinical & patient app experience | Deeplink implementation between apps |
| Data integration with clinical systems | M2M web services API integration |
| Ensuring data adherence for RPM devices | Data acquisition reports to monitor adherence |
