Neurological Drug Safety
Continuous temperature monitoring for drug safety
Study Scope and Protocol
One of the primary indicators of patient safety and efficacy in this trial is changes in body temperature over extended periods.
This study leveraged Pulsebridge’s Biometric Data Platform the medical grade wearable temperature monitor to analyze patient temperature in ambulatory and at-home settings over a 3 week period.
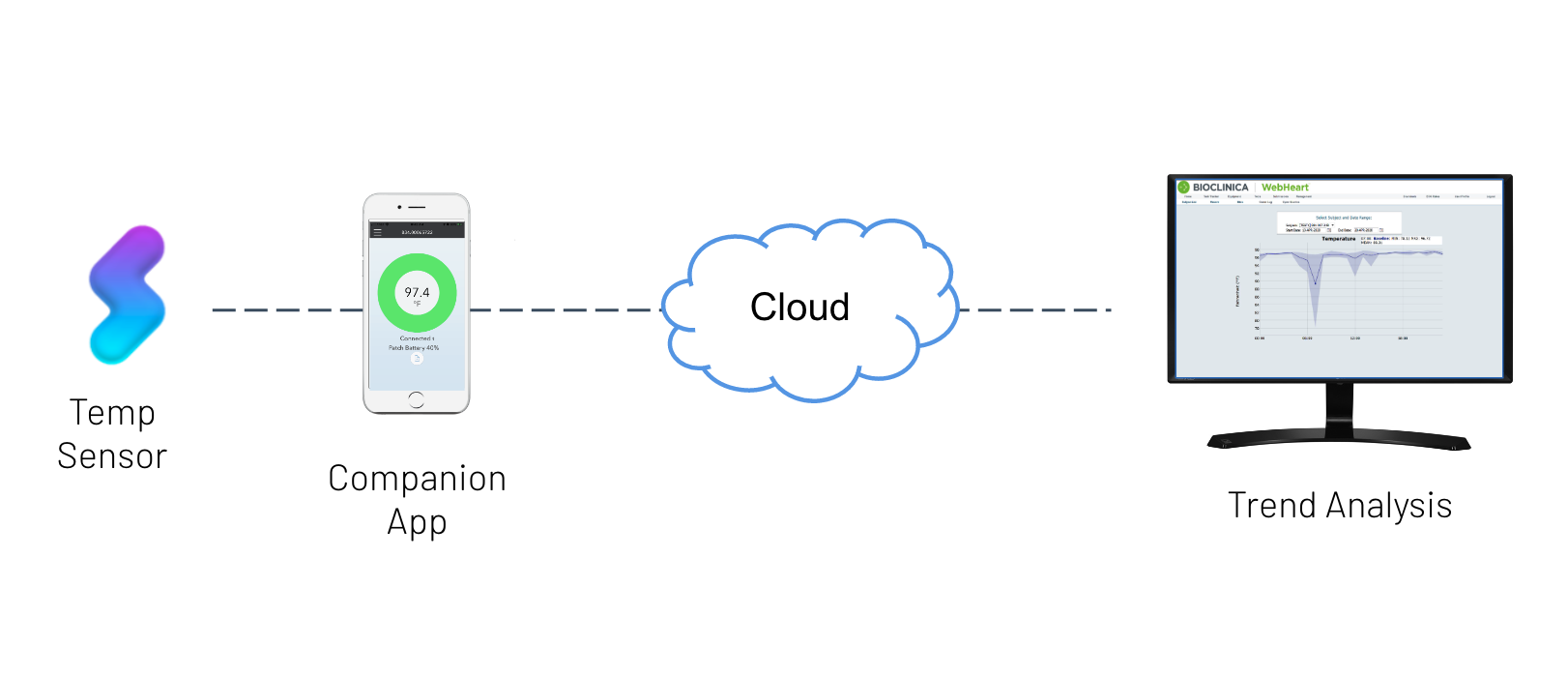
Key Components
- Biometrics Data Platform
- Wearable Temperature Monitor
- Custom Mobile Patient App
- Temperature Trend Analysis
- Clinical Systems Integration
Data Captured
- Axillary Temperature
| Requirements/Challenges | Solution |
| Ambulatory impacts to temperature variations | Trend analysis to flag artificial temperature changes |
| Extended monitoring period | 21 day rechargeable temperature sensors |
| Network disruptions in ambulatory settings | Sensors with onboard memory and automated network data synchronization |
| Data integration with clinical systems | M2M web services API integration |
